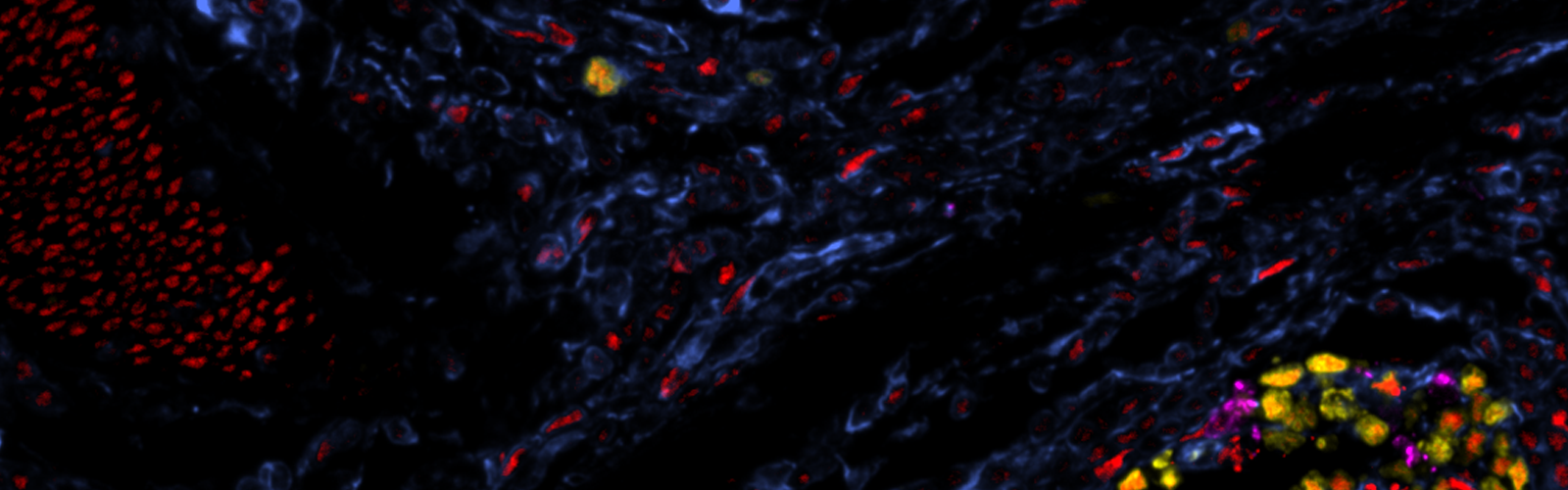
CST® Antibodies Work on the Cell DIVE Multiplex Imaging Solution
Multiplexing with Antibodies You Can Count On
The Cell DIVE Multiplex Imaging Solution by Leica Microsystems enables you to produce crystal-clear images of whole tissue using 60+ biomarkers, with automatic calibration and correction to enable robust downstream analysis.
Generating high-quality multiplexed images for spatial biology requires antibodies you can trust to perform consistently and accurately.
Why choose CST antibodies for Cell DIVE imaging?
CST and Leica Microsystems verified over 200 CST antibodies—testing a growing list of conjugated antibodies for Cell DIVE imaging to figure out the optimal experimental conditions, so you don't have to.
Save valuable time—avoid the hassle and cost of building, optimizing, and troubleshooting your antibody panel. Our antibody conjugates are developed using best-in-class CST antibodies that have been validated for immunohistochemistry (IHC) with confirmed sensitivity and specificity, so you can be confident in your results.
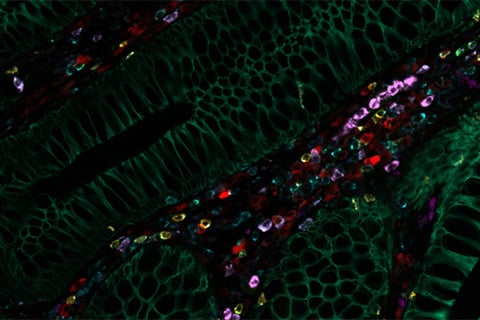
Normal tissue adjacent to colon adenocarcinoma: Tissue was iteratively stained with over 30 CST antibodies and imaged using the Cell DIVE Multiplexed Imaging Solution. Seven biomarkers are shown: GZMB (olive green), PanCK (dark green), CD45 (blue), CD8 (yellow), CD79A (red), CD68 (dark purple), CD11B (violet).
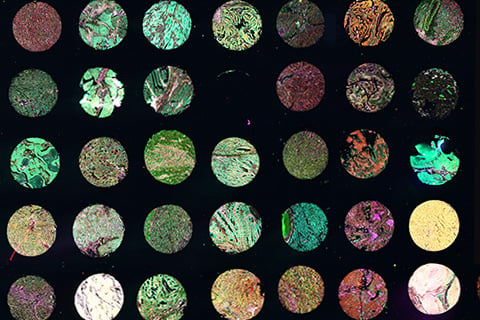
Multiple cancerous and normal tissue types were iteratively stained with multiple CST antibodies and imaged using the Cell DIVE Multiplex Imaging Solution. Optimal intensity values for all biomarkers are shown in one image.
How to Select CST Antibodies for Cell DIVE Imaging
Quickly tailor an antibody panel to suit your needs—choose from the catalog of CST antibody conjugates that are compatible for use with the Cell DIVE Multiplex Imaging Solution. Over 200 ready-to-ship antibodies and custom-conjugated antibodies are available upon request. Antibodies recommended for Cell DIVE imaging can be conjugated to Alexa Fluor® 488, Alexa Fluor® 555, Alexa Fluor® 647, and/or Alexa Fluor® 750. For more information, please contact your local sales representative.
Please note that Leica Microsystems has found that a final concentration of 1-4 μg/mL is optimal for staining, which typically requires a dilution of 1:50-1:200 for many of our antibodies. However, careful titration of the antibody conjugate may be necessary to achieve optimal signal and specificity in your sample.
Antibodies are also available in carrier-free formulations (without BSA or azide), should you need to conjugate an antibody to other fluorophores.
Custom Products Validated for Cell DIVE
Quickly customize an antibody panel to suit your needs—choose from the expanding list of antibodies that have already been custom-conjugated to common fluorophores and shown to work on the Cell DIVE Multiplexed Imaging System by Leica Microsystems. Please note that Leica determined that a final concentration of 1-4 ug/ml works most often for staining, which means a dilution of 1:50-1:200 works best for most of our antibodies. Careful titration of this antibody may be required to obtain optimal signal and specificity. Please reach out to your local sales representative to request more information. These antibodies are also available in carrier-free formulations (without BSA or azide) should you need to conjugate them to other fluorophores.
Need help getting the right conjugated antibody for your panel?
Expert CST scientists are ready to provide consultation on the best options for your antibody panels and can assist you with custom antibody conjugations. Don't know which ones to choose, or can’t find the antibody conjugate you need? We can help you. If you would like to order custom conjugation services, please fill out the Custom Antibody Conjugation Inquiries Form. With our support, you can get the multiplex images and data you need to move your discovery research forward.
View Research Posters by CST and Leica Microsystems
Spatial Proteomic Analysis of Immune Cells in Alzheimer's Disease Human Brain using Multiplexed Imaging and AI-assisted Phenotyping
Arindam Bose, Richard W. Cho, Sophie Struble, Gabriella Spang, Richard A. Heil-Chapdelaine, Natasha Fernandez Diaz Granados, Vasundhara Agrawal 5.9 MB PDF
AI-Powered Spatial Proteomics Analysis Reveals a Diverse Immune Landscape in Syngeneic 4T1 Murine Tumor Model
Arindam Bose, PhD, Emily Quann Alonzo, PhD, Sophie Struble, Gabriella Spang, Richard A. Heil-Chapdelaine, Natasha Fernandez Diaz Granados, Michael Smith, PhD, Matthew Norton, Samuel Jensen, PhD, Vasundhara Agrawal, PhD 5.9 MB PDF
Spatial Resolution of Immune Cell Lineages in the Tumor Microenvironment of Plasma Cell Dyscrasias
Medbh A. Dillon, Lisa Arvidson, Cole G. Phalen, Ruoxin Li, Imran McGrath, Julian R. Ishibashi, John B. Johanneson, Kevin J. Li, Zachary J.Thomson, Samuel Jensen, Jocelin Malone, Mackenzie S. Kopp, Susan A. Lundmann, Adam K. Savage, Claire E. Gustafson, Marla Glass, Emma L. Kuan, Lucas T. Graybuck, Xiao-jun Li, Troy R. Torgerson, Peter J. Skene, Stephanie Añover-Sombke, Melinda L. Angus-Hill 5.9 MB PDF
Vasundhara Agrawal , Lisa Arvidson, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Samuel Jensen, Arindam Bose 4.1 MB PDF
Vasundhara Agrawal, Lisa Arvidson, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Samuel Jensen, Arindam Bose 2.3 MB PDF
Spatial Architecture of Tumor and Immune Cell Lineages in Syngeneic Mouse Tumor Tissues
Arindam Bose, Lisa Arvidson, Emily Quann Alonzo, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Gabriella Spang, Matthew Norton, Samuel Jensen, Vasundhara Agrawal 4.5 MB PDF
Spatial Proteomic Analysis of Alzheimer's Disease Human Brain Using Multiplexed Imaging
Arindam Bose, Lisa Arvidson, Richard W. Cho, Supriya Singh, Gabriella Spang, Michael J. Smith, Vasundhara Agrawal 2.4 MB PDF
Lisa Arvidson, Reginaldo Prioli, Samuel Jensen, James B. Hoying, Michael W. Golway, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Hideki Sasaki, Chi-Chou Huang, Tuan H. Phan, Melinda L. Angus-Hill 24 MB PDF
Lisa Arvidson, Reginaldo Prioli, Samuel Jensen, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Hideki Sasaki, Chi-Chou Huang, Tuan H. Phan, Melinda L. Angus-Hill 25.2 MB PDF
Vasundhara Agrawal, Richard W. Cho, Sophie Struble, Jeremy Fisher, Gabriella Spang, Shelby Sponaugle, Richard A. Heil-Chapdelaine, Natasha Fernandez Diaz Granados, Arindam Bose
Vasundhara Agrawal, Emily Quann Alonzo, Sophie Struble, Jeremy Fisher, Richard A. Heil-Chapdelaine, Natasha Fernandez Diaz Granados, Arindam Bose
Multiplexed AI-powered Spatial Proteomics Maps Predictors of Immunotherapy Response in Breast Cancer
Vasundhara Agrawal, Emily Quann Alonzo, Sophie Struble, Jeremy Fisher, Richard A. Heil-Chapdelaine, Natasha Fernandez Diaz Granados, Arindam Bose
Reginaldo Prioli, Lisa Arvidson, Sam Jensen, Michael J. Smith, Katie O. White, Richard A. Heil-Chapdelaine, Hideki Sasaki, Chi-Chou Huang, Tuan H. Phan, Melinda L. Angus-Hil